Achievements
Discovery and development of statins
Atherosclerotic vascular diseases, including myocardial infarction and cerebral thrombosis, are the leading causes of death in the world. Increased levels of blood cholesterol are one of the major risk factors for atherosclerosis, and have been the major target of prevention and treatment of these life-threatening diseases. In 2014, WHO reported that ischaemic heart disease and stroke have remained the top 2 major killers in the world.1)
Discovery of compactin: In the early 1970s, in search of HMG-CoA reductase inhibitors in order to develop a cholesterol-lowering drug, we formed two hypotheses that (1) plasma cholesterol levels could be lowered more effectively by inhibiting hepatic HMG-CoA reductase, the rate-limiting enzyme in cholesterol biosynthesis, than by decreasing absorption from the diet and (2) some fungi would produce antibiotics that inhibited HMG-CoA reductase, possibly as a defense mechanism against other microbes that required sterols and/or other mevalonate derived isoprenoids for their growth. Based upon these hypotheses, we examined 6000 culture extracts of fungi and in July 1973, discovered ML-236B,2,3) now known as compactin or mevastatin from the culture extracts of Penicillium citrinum Pen-51(Fig.1) as a potent inhibitor of HMG-CoA reductase.
 |
 |
 |
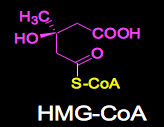
(Left) Fig. 1. Penicillium citrinum Pen-51. (Center) Fig.2. HMG −CoA.
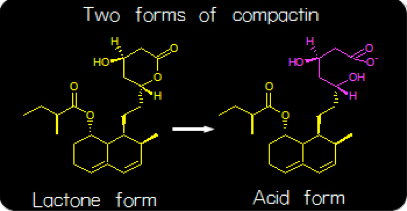
(Right) Fig. 3. The lactone form of compactin is converted to the acid form when ingested.
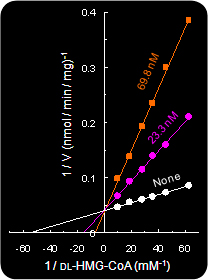
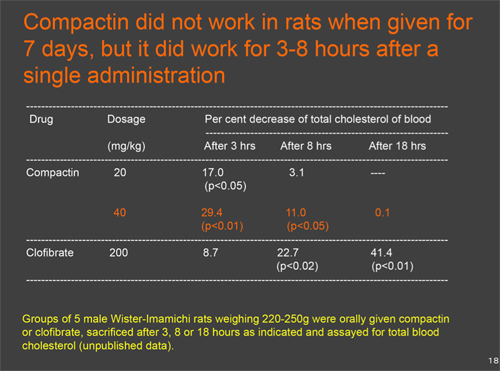
Mechanism of action in compactin: The biochemical mechanism of action in compactin was, as suggested by its structural similarity to HMG-CoA (Figs. 2 & 3), the substrate of HMG-CoA reductase reaction, that of a competitive inhibitor (Fig. 4).3) When given orally to rats, compactin selectively inhibited cholesterol synthesis in the liver, as compared to that in other organs.4) At very high concentrations, at which sterol synthesis and HMG-CoA reductase were strongly inhibited, cells were not able to grow and died, even in the presence of lipoproteins. This inhibition was overcome and cells grew normally by adding a small amount of mevalonate, the product of the HMG-CoA reductase reaction, indicating that inhibition by compactin was very selective to HMG-CoA reductase.5) Compactin was not able to lower serum cholesterol of rats when repeatedly given.6) However, compactin was able to reduce serum cholesterol of rats by 29.4% (p<0.01) after 3 hours when given as a single administration at 40mg/kg (Table 1, unpublished data). After 18 hours of single administration at 40 mg/kg, no reduction was seen in serum cholesterol even when compactin was repeatedly given to rats for 6 days, since hepatic HMG-CoA reductase was induced 10.4 fold as compared with control animals.6) After 18 hours of single administration at 40 mg/kg, no reduction was seen in serum cholesterol even when compactin was repeatedly given to rats for 6 days, since hepatic HMG−CoA reductase was induced 10.4 fold as compared with control animals.6) It was concluded that this strong induction of hepatic HMG-CoA reductase explained why compactin did not work when given repeatedly to rats. However, compactin was able to lower raised serum cholesterol in rats when both hepatic HMG-CoA reductase and higher levels of serum cholesterol were induced by an intravenous injection of the detergent Triton WR-13397). These results suggested that compactin would be effective in other animal species and patients with elevated blood cholesterol levels.
 |
 |
(Left) Fig.4. Competitive inhibition of HMG-CoA reductase by compactin.
(Right) Table1. Cholesterol-lowering effects of compactin in rats after a single administration.
Lowering LDL cholesterol in animals: As an animal model with elevated blood cholesterol levels was not available, we selected laying hens that produced an egg every day, which contained high cholesterol. Experiments showed that compactin strongly reduced blood cholesterol in laying hens nearly 50% after one month (unpublished data). Further, it was shown that compactin reduced serum cholesterol by 42% (on average) in dogs at 50 mg/kg for 13 days and 36% in monkeys at 50 mg/kg for 11 days, without severe adverse effects(Fig. 5). LDL cholesterol was selectively lowered in these animals.8,,9)
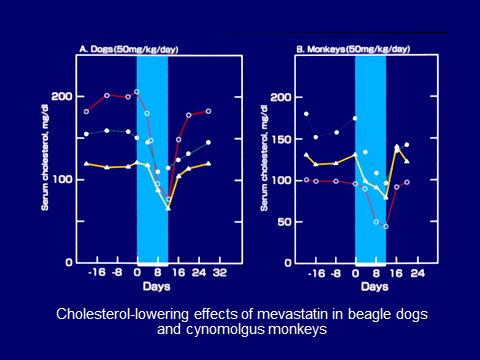 |
Fig. 5. Cholesterol-lowering effects of compactin in dogs (left) and monkeys (right).
Clinical trials: In February 1978, in collaboration with Akira Yamamoto, a physician at the Osaka University Hospital in Osaka, we first treated an 18-year old woman with familial hypercholesterolemia(FH) homozygote (Fig. 6). Her LDL cholesterol dropped from 1,000 mg/dl to 700 mg/dl during treatment with compactin at 500 mg/day for 2 weeks.10) In the following 6 months, we treated 8 patients with severe hypercholesterolemia including FH heterozygotes for 6-8 weeks at 50-100 mg/day and their plasma cholesterol declined by 28% on average with no severe side effects10). In November 1978, Sankyo started a phase 1 clinical trial for compactin. In phase 2 of the trial, started in the summer of 1979, compactin was administered to subjects with serious cases of hypercholesterolemia at 12 hospitals in Japan. All of the participating hospitals reported positively on the remarkable efficacy and excellent safety profile of compactin. In those clinical trials, compactin reduced plasma LDL cholesterol by 20-40% at 30-60 mg/day and LDL cholesterol was lowered by 50-60% by combining with a bile acid sequestrant.11,12) In August 1980, however, Sankyo suspended the clinical development of compactin, because it caused lymphoma in dogs that received 100 and 200 mg/kg/day for 2 years, while no abnormality was noted in the group receiving 20 mg/kg/day for 2 years.13) The same trouble was avoided in the development of pravastatin, second statin at Sankyo, by limiting its maximum dose to 25mg/kg/day, excluding 100 and 200 mg/kg/day doses.13,14)
 |
Fig. 6. The woman who was first treated with satins. Here, she is holding her baby 7 years after the treatment.
Development of compactin analogs, collectively called statins
However, the importance of our discovery of compactin was not lost on the pharmaceutical industry. Merck was first out of the box with lovastatin. From July 1976 to October 1978, Sankyo offered Merck crystals of compactin and its pharmacological and toxicological data under a “Disclosure Agreement“. In Feb 1979, Endo and Merck independently isolated lovastatin, the second statin.15-18) Merck developed lovaststatin while Sankyo did not develop it. The long-term toxicity studies in animals showed no tumors at Merck. In November 1986, Merck sent the New Drug Application (NDA) to the U.S. FDA and lovastatin was given FDA approval to become the first commercial statin in 1987.
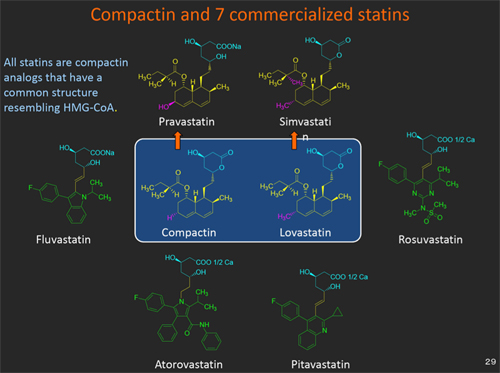 |
Fig. 7. Compactin and 7 commercial statins.
Since lovastatin was commercialized, 6 more statins, including 2 semi-synthetic statins (pravastatin and simvastatin) and 4 synthetic statins (fluvastatin, atorvastatin, rosuvastatin and pitavastatin) have been introduced to the market (Fig. 7).19) Lovastatin was followed by a new statin, simvastatin at Merck. Sankyo, in turn, developed pravastatin and launched it in 1989. Four other types of synthetic statin were subsequently developed. All commercial statins are compactin-analogs that have a common structure resembling HMG-CoA.3,19)
Large-scale clinical trials of statins
Statins have been tested in 14 large randomized multicenter trials, involving an unprecedented 90,056 middle-aged adults who were followed for five years.20) The results in all 14 studies were astonishingly consistent: treatment with statins lowered plasma LDL by 25-35% and reduced the frequency of heart attacks by 25-30%. The percentage reduction in coronary events would be even more dramatic if the treatment were longer and if statin therapy were started earlier when clinically silent atherosclerotic plaques are fewer and smaller. A noteworthy aspect of the 14 statin trials is that no major harmful effects of lowering cholesterol were observed in any of the studies. The remarkable safety of statins derives from their unique mechanism of action. When statin is ingested, the drug is routed primarily to the liver where it inhibits HMG CoA reductase, lowering cholesterol production. This decrease in liver cholesterol triggers a compensatory feedback loop that increases the number of LDL receptors displayed on the liver cell membrane. These LDL receptors grab onto LDL, and remove it from the blood.21) At present, statins are the largest-selling class of drugs currently taken by patients throughout the world. Sales for this one class of drugs in 2005 were $25 billion.22) Today, an estimated 40 million people worldwide are taking statins.
2013 ACC/AHA guideline to lower blood cholesterol: In November 2014, the American College of Cardiology and the American Heart Association announced the 2013 ACC/AHA guidelines on the treatment of blood cholesterol, in which they recommended 4 groups of individuals to take statins:23) (1) individuals with clinical ASCVD (atherosclerotic cardiovascular disease), (2) individuals with primary elevations of LDL-C ≥190 mg/dl, (3) individuals 40 to 75 years of age with diabetes and LDL-C 70 189 mg/dl without clinical ASCVD and (4) individuals without clinical ASCVD or diabetes who are 40 to 75 years of age and have LDL-C 70 to 189 mg/dl and an estimated 10-year ASCVD risk of ≥7.5%. This requires a clinician-patient discussion22).
References
- 1) WHO. The 10 leading causes of death in the world, 2000 and 2012.
- 2) Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new Inhibitors of cholesterogenesis produced by Penicillium citrinum. J Antibiot 1976; 29:1346-1348.
- 3) Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl- coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett 1976; 72: 323-326.
- 4) Endo A, Tsujita T, Kuroda M, Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3- methylglutaryl-coenzyme A reductase. Eur J Biochem 1977; 77: 31-36.
- 5) Kaneko I, Hazama-Shimada Y, Endo A. Inhibitory effects on lipid metabolism in cultured cells of ML-236B, a potent inhibitor of 3-hydroxy-3-methylglutaryl- coenzyme-A reductase. Eur J Biochem 1978; 87: 313-321.
- 6) Endo A, Tsujita Y, Kuroda M, Tanzawa K. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta 1979; 575: 266-276.
- 7) Kuroda M, Tanzawa K, Tsujita Y, Endo A. Mechansim for elevation of hepatic cholesterol synthesis and serum cholesterol levels in Trition WR-1339-induced hyperlipidemia. Biochim Biophys Acta 1977; 489: 119-125.
- 8) Tsujita Y, Kuroda M, Tanzawa K, Kitano N, Endo A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis 1979; 32: 307-313.
- 9) Kuroda M, Tsujita Y, Tanzawa K, Endo, A. Hypolipidemic effects in monkeys of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids 1979; 14: 585-589.
- 10) Yamamoto A, Sudo H, Endo A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis 1980; 35: 259-266.
- 11) Mabuchi H et al. Effects of an Inhibitor of 3-hydroxy-3-methylglutaryl coenzyme a reductase on serum lipoproteins and ubiquinone-10 levels in patients with familial hypercholesterolemia. New Engl J Med 1981;305: 478-482.
- 12) Mabuchi H et al. Reduction of serum cholesterol in heterozygous patients with familial hypercholesterolemia ― Additive effects of compactin and cholestyramine. New Engl J Med 1983;308: 609-613.
- 13) Outline of CS-500 (ML-236B). Conference on the clinical trials phase II of CS-500 (ML-236B). Sankyo Co., Tokyo. August 22, 1979 and testimonies of researchers and physicians involved in the development of compactin.
- 14) Mevalotin Interview Form (11th revised edition ) (in Japanese). No.872189. Sankyo Company, Tokyo. October 2015. p.42.
- 15) Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monasus species. J Antibiot 1979; 32: 852-854.
- 16) Endo A. Monacolin K, a new hypocholesterolmic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Antibiot 1980; 33: 334-336.
- 17) Alberts AW et al. Mevinolin: a highly potent competitive inhibitor of hydroxymethyl- glutaryl-coenzyme A reductase and a cholesterol- lowering agent. Proc Natl Acad Sci USA 1980;77:3957-3961.
- 18) Vagelos PR. Are prescription drug prices high? Science 1991; 252:1080-1084,.
- 19) Endo A. A historical perspective on the discovery of statins. Proc Jap Acad, Ser B 2010; 86: 488 493.
- 20) Baigent C et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267-1278.
- 21) Award presentation by Joseph Goldstein. The 2008 Lasker-DeBakey Clinical Medical Research Award. Statins for lowering LDL and decreasing heart attacks by Akira Endo.
- 22) Gray N. A special report on the world’s top 50 pharma companies. Article-354138 pdf-Adobe Reader. May 2005.
- 23) The 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889-2934.